Russian Journal of Coordination Chemistry, Vol. 23, No. 3, 1997, pp. 177-181. Translated from Koordinatsionnaya Khimiya, Vol. 23, No. 3, 1997, pp. 192-196. Original Russian Text Copyright � 1997 by Blatov, Serezhkin.
Structural and Topological Features of Oxygen-containing Zirconium(IV) Compounds
V. A. Blatov and V. N. Serezhkin
Samara State University, Samara, Russia
Received February 23,1996
Abstract — Structural and topological studies of the coordination environment of Zr(IV) atoms in oxygen-containing crystal compounds are carried out by means of the Voronoi-Dirichlet polyhedrons (VDPs). A rigorous method of determining the combinatorial-topological type of coordination polyhedron (CP) consists of analyzing the appropriate dual VDP. To account for the distortions of the coordination sphere caused by the thermal motion of atoms, dynamical VDPs and CPs are introduced. It is shown that, for high coordination numbers of a zirconium atom (C.N. � 6), the volume of ZrOn VDP is virtually independent of C.N. and equals 9.04(6) µ3. The oxygen-containing sublattices of the compounds studied are found to be topologically similar to the aperiodic systems that model fluids.
In [1], we studied the geometric features of the Voronoi-Dirichlet polyhedrons (VDPs) for zirconium(IV) atoms in structures of simple and complex fluorides having the coordination polyhedrons (CPs) of ZrFn (n = 6, 7, or 8) composition. It was established that the volume of the VDPs of zirconium atoms is virtually independent of their coordination number (C.N.). An analogous fact was previously established only for the U(IV-VI) atoms, which form the UOn CPs with n ranging from 6 to 12 in the structure of oxygen-containing compounds [2, 3]. It was of interest to find out how modification of the chemical nature of the surrounding atoms influences the characteristics of VDP for a given type of complexing atom. With this in mind, we have undertaken the study of the geometry and topology of the ZrOn CPs and corresponding VDPs using the methods of topological analysis described in [4].
From the crystallographic database for the oxygen-containing Zr(IV) compounds, 92 compounds were chosen whose structures contained 112 crystallographically independent zirconium atoms and satisfied the following conditions: (1) the coefficient of divergence did not exceed 10% for the structural experiment, and (2) there was no statistical disorder in the arrangement of the zirconium and (or) oxygen atoms comprising the ZrOn CP in the structure.
An analysis of the VDPs (Table 1) indicates that the C.N. of Zr(IV) atoms in the oxygen environment ranges from 4 to 9, although C.N. 6 and the octahedral CP are most typical for Zr(IV). An increase in the C.N. of zirconium atoms and the respective increase in the number of VDP faces lead to a monotonic decrease in its surface area and in the G3 value characterizing the degree of uniformity of the zirconium environment [4], thereby indicating that the VDP shape approaches a sphere. As in the previously studied uranium and zirconium coordination compounds, the volume of the VDP of complexing atom with a C.N.
� 6 virtually does not change and is equal to 9.04(6) µ3 However, the fact that the VDP volume is considerably greater for C.N. 4 and 5 may be due to either one of the following: (1) to a profound difference in the electronic structures of the zirconium complexes having C.N. < 6 and C.N. � 6, or (2) to the errors that are introduced by the chosen dividing factor Kd [2] because not only oxygen atoms contribute to the formation of VDP at C.N. 4 or 5. Unfortunately, we failed to determine which one of these was the cause because of the low number of the known zirconium compounds having C.N. 4 or 5 (compounds 1 and 2, respectively).As was already pointed out in [1-3], the VDPs of atoms A that are chemically bonded to the surrounding atoms X are characterized by the linear relationships between the solid angles (
Wi) of the VDP faces corresponding to the A-X contacts and the relevant interatomic distances, i.e.,W
i(A-X) = A - Bri(A-X) (1)ln
Wi(A-X) = C - Dri(A-X). (2)The data obtained indicate that the VDPs of composition ZrOn obey relationship (2), namely,
ln
Wi(Zr–O) = 6.64(5) - 1.85(2) ri(Zr–O), (3)with the coefficient of correlation equal to -0.95 for 752 experimental points. Note that the analogous dependence for the Zr-F contacts in ZrFn CPs [1] is characterized by nearly the same values of the coefficients Á and D [6.67(5) and -1.95(2), respectively]. In our opinion, this fact is evidence for the similarity of the types of Zr-O(F) interactions in the coordination compounds. Note parenthetically that, although the
volume of the VDP of composition ZrXn, with X = F [1] or O (Table 1), is virtually independent of the C.N. of Zr(IV) for n � 6, it significantly increases (by � 1 µ3) on passing from ZrFn to ZrOn, with a simultaneous increase in Rsd from 1.24(1) to 1.29(1) µ. In spite of the fact that we did not use any atomic radii (for Zr, F, O, and others) in calculating the geometric characteristics of VDPs, the difference obtained Rsd(ZrOn) - Rsd(ZrFn) = 0.05 µ is close to the difference in the known radii [6] R(O2-) – R(F–) � 0.06-0.07 µ.Table 1. Geometric characteristics of the Voronoi-Dirichlet polyhedrons of a zirconium atom*
C.N. of zirconium |
Number |
VVDP, µ3 |
SVDP, µ2 |
G3 � 103 |
Rsd , µ |
|
of compounds |
Of atoms |
|||||
4 |
1 |
1 |
12.9 |
37.8 |
100 |
1.46 |
5 |
2 |
2 |
11.2(9) |
32(4) |
92(10) |
1.39(4) |
6 |
52 |
63 |
9.09(9) |
26.3(2) |
84.1(2) |
1.294(5) |
7 |
10 |
11 |
9.0(2) |
25.3(3) |
82.5(6) |
1.292(7) |
8 |
25 |
30 |
8.96(9) |
24.6(2) |
81.6(4) |
1.288(4) |
9 |
2 |
2 |
8.9(8) |
23.9(2) |
80(5) |
1.29(4) |
6, 7, 8, and 9 |
89 |
109 |
9.04(6) |
25.6(2) |
83.1(3) |
1.292(3) |
* VVDP, SVDP, and Rsd are the volume of VDP, its surface area, and radius of the sphere of volume equal VVDP, respectively. Bracketed are the confidence intervals for the average values corresponding to the fiducial probability of 95%. A noncoincidence of the total number of compounds in the second column with that indicated in the text is due to the occurrence of different C.N. for the zirconium atoms in the structure of Zr(OH)2CrO4 [5].
A rigorous determination of the combinatorial-topological type of CP was carried out by searching for the isomorphism of the adjoint graphs of the corresponding dual VDPs using the slightly modified IZOM program [7]. Considering that the knowledge of the combinatorial type of CP does not provide exhaustive information about the geometry of the surroundings of the central atom, because even weak distortion of the CP's metric can change its combinatorial type, the ultimate assignment of CP to a particular combinatorial type was performed using the concept of a dynamical VDP that is presented below.
In describing the topology of a many-body system, account was taken of its dynamics using the concept of a Delone dynamical system as an ensemble of the Delone static systems (as defined in [9]). The crystal lattice can be regarded as the Delone dynamical system as well, provided that the atomic vibrations are taken into account. The structural-topological properties of the nearest surroundings of the p, atom can be naturally characterized by introducing the dynamical VDPs [PD(pi)] into the Delone dynamical system. PD(pi) is defined as a set [Pk(pi)] of the static VDPs belonging to the ensemble of Delone static systems, with the weight
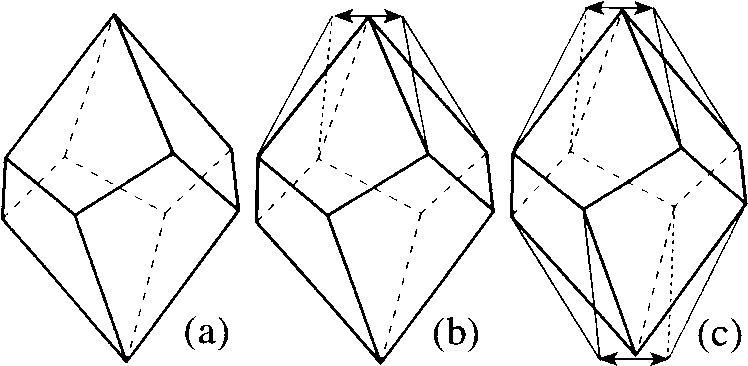
w k equal for each Pk(pi) to the probability of realizing Pk(pi) within the dynamical system. Thus, PD(pi) is a VDP averaged over all VDPs that are realized for pi in the moving system during a certain time interval. Let us call the maximum value of w k (w max) the degree of stability of PD(pi); PD(pi), for which (w max = 1, the stable dynamical VDP; and denote Pk(pi) with w k = w max by Pmax(pi). Obviously, the stable PD(pi) can occur only in the solid state, where the translational motion of atoms is virtually absent, while the power of the {Pk(pi)} set is limited. Therefore, the atom in the crystal lattice possesses the stable dynamical VDP, provided that the topology of its surroundings (and, hence, topology of CP) does not change in the course of the oscillatory motion.A dynamical CP [CPD(pi)] dual with the dynamical VDP can be introduced in a similar manner. CPD(pi) is a set of all CPk(pi) dual with the corresponding Pk(pi) from PD(pi). The data of the structural experiment are ordinarily invoked for determining the "average" PX-ray(pi) and CPX-ray(pi) polyhedrons, which, in general, differ from Pmax(pi) and CPmax(pi). Let us consider, as an example, the dynamical CP of the zirconium atom in the structure Zr(IO3)4 [10]. In this case, PX-ray(pi) and CPX-ray(pi) are the tetragonal trapezohedron (Fig. 1a) and tetragonal antiprism, respectively. At the same time, it is known that the VDPs having the (
n >3)-fold vertices (the so-called "partial" VDPs [11]) are combinatorially unstable to the arbitrary (infinitesimal) distortion of geometry. For this reason, PD(Zr) must include the polyhedrons that are formed from PX-ray(Zr) (Fig. 1a) after splitting one (Fig. 1b) or two (Fig. 1c) of its vertices having n = 4. The corresponding CPs relate to the combinatorial-topological types of bicapped trigonal prism and trigonal dodecahedron, respectively, although geometrically can be assigned to the type of a weakly distorted tetragonal antiprism. Since, among the zirconium eight-vertex CPs, only the trigonal dodecahedron is combinatorially stable (it corresponds to the "general", by definition [11], VDP with the vertices all having n = 3), for C.N. 8, this type of CP must be most often realized for CPX-ray(Zr), as is actually the case (Table 2). The corresponding PD(Zr) can include the partial VDPs, and, hence, among the trigonal-dodecahedral CPsX-ray(Zr), CPs can also occur that are geometrically close to the tetragonal antiprism or bicapped trigonal prism. Inasmuch as the general VDPs are dual with the general CPs having exclusively triangular faces, the known CP types can be divided into two groups: combinatorially stable (general) and combinatorially unstable (partial) polyhedrons. For instance, of the CPs considered in [12], the polyhedrons listed in Table 2 refer to the first group. The remaining combinatorial CP types are partial and must occur in the crystal structures much more rarely.Fig. 1. Transformation of (a) VDP corresponding to the CP in the form of a tetragonal antiprism into the VDP corresponding to the CP in the form of (b) a bicapped trigonal prism and (c) trigonal dodecahedron. Arrows indicate the directions in which the VDP vertices are split. The transformed parts of VDP are indicated by fine lines.
Therefore, the known problem of coordination chemistry concerning the assignment of the CP to a certain geometric type can be reformulated in terms of the concept of a dynamical VDP, and the known geometric criterions that are used in classification of the eight-vertex CPs [13] can be generalized and extended to the CPs having an arbitrary number of vertices. The combinatorially stable (general) CP(pi) can be considered as the geometrically distorted variant of one of the combinatorially unstable (partial) types, provided that the corresponding partial VDP is contained in PD(pi). To obtain the set of partial VDPs, one should successively constrict all the edges of a general VDP that are shorter than a given limiting length, which can be reasonably chosen to be equal to the error in determining the geometric parameters of relevant atoms by the structural experiment. Note that constriction of the VDP edge is equivalent to the transformation of the appropriate CP dihedron into monohedron, so that the suggested procedure of searching for the partial CPs is analogous to the use of a dihedral-angle criterion [13] and generalizes it to the arbitrary polyhedrons.
Table 3 presents the results of applying the described procedure in the combinatorial CP analysis of ZrXn (X = O or F). As a criterion for constricting the VDP
r edge, the condition a r <3s � 1� was chosen, where a r is the angle at which the edge r is "seen" from the central atom and a is the error in determining the OZrO angles in the structure. The data in Table 3 indicate that 15 combinatorial types exist for ZrXn (n = 4, 5, 6, 7, 8, or 9) CPsX-ray(Zr), of which the octahedron, trigonal dodecahedron, and pentagonal bipyramid are most abundant. CPsX-ray(Zr), as a whole, are combinatorially stable to the distortions caused by the oscillatory motion of atoms, although, in some cases, PD(Zr) and CPD(Zr) contain several combinatorial-topological polyhedron types. A typical example is the Zr(SO4)2Ç 4H2O structure [14], in which CPsX-ray(Zr) is a trigonal dodecahedron, although it becomes geometrically close to the tetragonal antiprism if the oscillatory motion of the atoms is taken into account.Fig. 2. Frequency (in percent of sample size n) with which the VDP with a given number of faces occurs in the sample containing the VDPs for the zirconium sublattice in the crystal structures of (a) oxygen-containing coordination compounds (n= 112) and (b) coordination compounds with organic ligands (n = 709) [15]. For each of the histograms, one step in abscissa corresponds to changing the number of VDP faces by unity. The columns corresponding to tetradecahedrons are shaded.
Considering that the CPs can be distorted not only by the lattice dynamics and other factors influencing the accuracy of the structural experiment, but also by other causes, primarily by the peculiarities of the electronic structure of complexes, we have studied the combinatorial stability of CPX-ray(Zr) to more severe distortions than those satisfying condition
a r <3s . Table 3 presents the results of analyzing the combinatorial characteristics of the ZrXn (X = O or F) polyhedrons obtained from CPX-ray(Zr) after constricting the edges satisfying condition a r <a lim (a lim = 5�, 10�, 15�, 20�, or 30�). An analysis of the data indicates that the CPs that are typical for C.N. 6 or 7 are topologically fairly stable up to a r = 30� (i.e., their number in the sample either remains unchanged or increases by virtue of the appropriate transformations of the CPs of other combinatorial types), whereas part of the trigonal-dodecahedron-shaped CPs transforms into the bicapped trigonal prisms or tetragonal antiprisms even at a r < 1�. Note that the ZrF9 CPs in the form of a tricapped trigonal prism are low-stable and disappear from the sample at a r > 10� (Table 3). This fact is consistent with the classical idea that the C.N. of zirconium atoms in fluoro-containing compounds does not exceed 8. It is also consistent with the conclusion [1] that the small faces corresponding to the nonvalence Zr–F interactions are present in all ZrF9 CPs.
Table 2. General combinatorial-topological types of coordination polyhedrons
C.N. |
Combinatorial-topological type |
4 |
Tetrahedron |
5 |
Trigonal bipyramid |
6 |
Octahedron |
7 |
Monocapped octahedron |
Pentagonal bipyramid |
|
8 |
Trigonal dodecahedron |
9 |
Tricapped trigonal prism |
10 |
Bicapped tetragonal antiprism |
Quadricapped trigonal prism |
|
12 |
Icosahedron |
Fig. 3. Frequency (in percent of sample size n) with which the VDP with a given number (f) of faces occurs in the sample (n = 723, solid line) containing the VDPs for the oxygen sublattice in the crystal structures of oxygen-containing zirconium coordination compounds, and sample (n = 10000, dashed line) containing the VDPs for the points of the ideal-gas system [16].
Figure 2a and Table 4 present the results of the topological analysis of the zirconium sublattices for the structures studied. Similar to the sublattices of other complexing atoms [15], the mutual arrangement of the zirconium atoms obeys the fourteen-neighbor rule and is characterized by the dominance of the VDPs in the form of a Fedorov's cubooctahedron {4/6 6/8} (Table 4). A comparison with the zirconium sublattices in its coordination compounds with organic ligands [15] (Fig. 2b) indicates that, in the structures of inorganic compounds, the fraction of dodecahedral and octahedral VDPs is significantly (3.5 and 35 times for dodecahedrons and octahedrons, respectively) larger, as was already pointed out for the organic and inorganic uranium coordination compounds [15]. This fact evidences a higher rigidity of the ZrOn CPs in the inorganic zirconium compounds than in the organic ones, in accordance with the model of deformed spheres [15].
The number-of-face (f) distribution [FVDP(f)] of VDPs for the oxygen sublattice also obeys the fourteen-neighbor rule, although, in this case, the distribution function (Fig. 3) is essentially different: it resembles the FVDP(f) function for a model "ideal-gas" system considered in [16], though differs by the position of the maximum at f = 14. According to [17], this fact is evidence for a considerable interaction between the oxygen atoms in the structures considered and for the topological similarity of the oxygen-containing sublattices to the aperiodic systems modeling fluids.
Table 3. Combinatorial-topological analysis of the Voronoi-Dirichlet and coordination polyhedrons of zirconium
VDP* |
CP |
X** |
Number of polyhedrons with a r � *** |
||||||
0� |
1� |
5� |
10� |
15� |
20� |
30� |
|||
{4/6} |
Octahedron |
O |
57 |
57 |
58 |
59 |
60 |
62 |
63 |
F |
17 |
17 |
17 |
17 |
17 |
17 |
17 |
||
{4/4 5/4} |
Trigonal dodecahedron |
O |
30 |
29 |
24 |
24 |
21 |
15 |
11 |
F |
36 |
34 |
32 |
26 |
23 |
23 |
13 |
||
{4/5 5/2} |
Pentagonal bipyramid |
O |
9 |
9 |
9 |
9 |
9 |
9 |
8 |
F |
14 |
14 |
14 |
14 |
13 |
12 |
11 |
||
{4/3 5/6} |
Tricapped trigonal prism |
O |
3 |
3 |
2 |
2 |
2 |
2 |
2 |
F |
4 |
3 |
2 |
1 |
0 |
0 |
0 |
||
{4/6 5/2} |
Bicapped trigonal prism |
O |
1 |
1 |
5 |
4 |
6 |
12 |
11 |
F |
3 |
4 |
5 |
10 |
12 |
11 |
13 |
||
{4/8} |
Tetragonal antiprism |
O |
1 |
2 |
3 |
4 |
4 |
4 |
8 |
F |
1 |
3 |
4 |
4 |
5 |
8 |
13 |
||
Other (9 types) |
Other (9 types) |
O |
11 |
11 |
10 |
10 |
10 |
8 |
9 |
F |
4 |
4 |
6 |
7 |
9 |
8 |
12 |
||
Notes: * In the notations for VDPs, the numerator of the fraction specifies the number of the face's vertices, and the denominator indicates the number of faces of a given type.
** Type of atoms X in the ZrXn CP.
*** The number of the sampled VDPs and CPs of a given type, as obtained after constricting all those VDP edges that correspond to the angle
ar smaller than the indicated limiting value.
Table 4. VDP distribution among the combinatorial-topological types for the zirconium sublattices*
VDP type |
Number of VDPs, % |
VDP type |
Number of VDPs, % |
{4/6 6/8) |
788 |
{3/8 4/2 6/4 10/4} |
3.6 |
(4/4 5/4 6/6}-1 |
8.1 |
(4/12} |
3.6 |
(4/6 6/2} |
8.1 |
{4/6} |
3.6 |
{4/8 6/4} |
3.6 |
* Indicated are the numbers of VDPs of a given type, in percent of the sample size n (n = 112); the data are presented only for those VDPs that occur with a frequency higher than 3%. In the notations for VDPs, the numerator of the fraction specifies the number of the face's vertices, and the denominator indicates the number of faces of a given type. The numbers beyond the brackets (they correspond to the notations adopted in [ 15]) permit one to distinguish between the polyhedrons whose types have the same notations.
ACKNOWLEDGMENTS
This work was supported by the Russian Foundation for Basic Research, project no. 95-03-08583.
REFERENCES
1. Serezhkin, V.N., Blatov, V.A., and Kukiina, E.S., Koord. Khim., 1996, vol. 22, no. 9, p. 145.
2. Serezhkin, V.N., Blatov, V.A., and Shevehenko, A.P., Koord. Khim., 1995, vol. 21, no. 3, p. 163.
3. Serezhkin, V.N., Shevehenko, A.P, and Blatov, V.A., Koord. Khim., 1996, vol. 22, no. 1, p. 76.
4. Blatov, V.A., Shevehenko, A.P., and Serezhkin, V.N., Acta Crystallogr., Sect. A: Found. Crystallogr., 1995, vol. 51, no. 6, p. 909.
5. Mark, W., Acta Chem. Scand., 1972, vol. 26, no. 19, p. 3744.
6. Shannon, R.D., Acta Crvstallogr., Sect. A: Found. Crystallogr., 1976, vol. 32, no. 5, p. 751.
7. Algoritmy i programmy resheniya zadach na grafakh i setyakh (Algorithms and Programs for Solving the Problems with Graphs and Networks), Nechepurenko, M.I., Ed., Novosibirsk: Nauka, 1990.
8. Blatov, V.A. and Serezhkin, V.N., Dokl. Akad. Nauk, 1995, vol. 343, no. 5, p. 637.
9. Galiulin, R.V, Kristallograficheskaya geometriya (Crystallographic Geometry), Moscow: Nauka, 1984.
10. Larson, A.C. and Cromer, D.T., Acta Crystallogr., 1961, vol. 14, no. I, p. 128.