Russian Journal of Coordination Chemistry, Vol. 22, No. 9, 1996, pp. 605-607. Translated from Koordinatsionnaya Khimiya, Vol. 22, No. 9, 1996, pp. 645-647. Original Russian Text Copyright � 1996 by Serezhkin, Blalov, Kuklina.
The Voronoi-Dirichlet Polyhedrons of Zr(IV) Atoms in Fluorine-containing Compounds
V. N. Serezhkin,
V. A. Blatov, and E. S. KuklinaSamara State University, Samara, Russia
Received November 17,1995
Abstract. The geometric characteristics of the Voronoi-Dirichlet polyhedrons are determined for 79 crystallographic types of Zr(IV) atoms whose positions in crystal structures are surrounded by fluorine atoms. The volumes of the Voronoi-Dirichlet polyhedrons are found to be virtually independent of the coordination number (6, 7, or 8) of the zirconium atoms, and their average value is 8.0(2) µ3.
When analyzing oxygen-containing uranium compounds, it was found that the volumes of the Voronoi-Dirichlet polyhedrons (VVDP) of the metal atoms forming the UOn coordination polyhedrons in the crystal structure are virtually independent of the coordination number (C.N.) at the fixed oxidation state of U atoms (IV, V, or VI) [1,2]. For example, VVDP equals 9.2(3) µ3 for 354 crystallographic types of U(VI) atoms, despite the fact that both the composition and shape of the coordination polyhedrons (UO6, UO7, or UO8) and the U-O interatomic distances in the first coordination sphere (1.6 to 2.7µ) significantly differ for these atoms [1]. In this connection, it is of interest to analyze the characteristics of the Voronoi-Dirichlet (VD) polyhedrons for the complexing atoms of different chemical nature that are capable (like the uranium atoms) of forming, in the crystal structure, coordination polyhedrons AXn with various n (i.e., C.N.A) and with the same nature of the X atoms in the first coordination sphere of the A atom.
In this work, the crystal compounds containing the Zr(IV) atoms surrounded by the fluorine atoms are chosen as the objects of investigation. As is known [3], the C.N. of Zr(IV) atoms with respect to the fluorine atoms is 6, 7, or 8, and the corresponding coordination polyhedrons are ZrF6 (octahedron), ZrF7 (as a rule, pentagonal bipyramid or monocapped trigonal prism), or ZrF8 (square antiprism, dodecahedron, or bicapped trigonal prism), respectively.
As primary information, we used the data on the crystal structure of 65 compounds that satisfy the following conditions: (a) their structures are determined with R
�0.10, and (b) Zr and/or F atoms are not statistically distributed over the positions of one or several regular systems of points. The sampled compounds involved 79 crystallographic types of the Zr(IV) atoms (17, 20, and 42 types with the C.N.s equal to 6, 7, and 8, respectively), and the geometric characteristics of their VD polyhedrons were calculated using a TOPOS set of structure-topological programs [4]. Note that 31 of the 65 chosen compounds contained the hydrogen atoms. Inasmuch as their positions were determined only for the structures of 15 compounds and the accuracy of determining the hydrogen coordinates, as a rule, is normally low, H atoms were disregarded when calculating the VD polyhedrons.According to the results obtained, the C.N. of the majority of Zr atoms, if taken equal to the total number of faces in the corresponding VD polyhedrons (C.N.VDP), coincided with the values given in the original works. At the same time, the VD polyhedrons of eight Zr atoms (four with C.N. 7 and four with C.N. 8) have one (for seven atoms) or two {only for the Zr(l) atom in the structure of Cs5Zr4F21
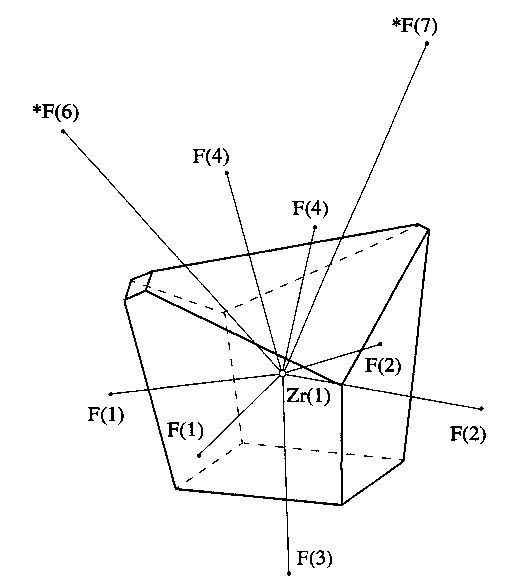
Ç3?2? [5]} additional small faces. All of these faces correspond to the small solid angles Wi [smaller than 1.5% of the total solid angle (471 sr) of the Zr atom] and abnormally long interatomic distances Zr–F (longer than 3 µ), so that the additional contacts can be considered, as in the case of the U(VI) compounds [1], as nonvalence interactions, the more so as, in most cases, they are related to the so-called indirect neighbors [6, 7], i.e., to those X, atoms (surrounding atom A) for which the A–X, sections do not intersect the corresponding faces of the VD polyhedron of atom A. As an example, Table 1 represents the main characteristics of the VD polyhedron of the Zr(l) atom in the structure of Cs5Zr4F21Ç 3?2?. Figure 1 shows the shape of the VD polyhedron. The atomic numbering in Table 1 and Fig. 1 is identical and coincides with that given in the original work [5].As is known, each interatomic A-Xi contact with the distance of ri(A–X) corresponds to a certain face of the VD polyhedron AXn, with the area Si of the face corresponding to a certain solid angle
Wi [1]. Note that, similar to the uranium compounds [2], zirconium fluorides exhibit a clear-cut dependence between the lnWi(A–X) and ri(A–X) values (Fig. 2), for which the linear regression analysis gives equationIn
Wi(Zr–F) = 6.67(5) – 1.95(2)ri(Zr–F) (1)
Fig. 1. The Voronoi-Dirichlet polyhedron of the Zr(l) atom in the Cs5Zr4F21Ç3?2? structure [5].
where the correlation coefficient
r = -0.963 for 581 faces of the VD polyhedrons of the zirconium atoms of 79 crystallographic types. In (1), the Wi values are expressed in percentages of the total solid angle (4p sr) of the zirconium atom, and the ri(Zr–F) values are given in angstroms. Note that six faces with Wi(Zr–F) < 1% were disregarded when deducing equation (1). The data [2] and the results obtained suggest that the correlations of the type (1) with r > 0.9 are valid only for those VD polyhedrons of composition AXn in which the strong chemical interaction between the atoms leads to the appearance of a short-range order in the mutual arrangement of the X atoms relative to the A atoms.In our opinion, the results of statistical analysis of the main geometric characteristics of the VD polyhedrons (Table 2) enable one to draw the following conclusions.
The volume of the VD polyhedron of the zirconium atom surrounded by the fluorine atoms in the crystal structure is virtually independent of the C.N. of the Zr(IV) atom and equals 8.0(2) µ3 for 79 crystallographic types of the VD polyhedrons.
The constancy of the VVDP value results in the constancy of the radius of the spherical domain (or interaction sphere) (Rsd) that corresponds to the sphere of volume VVDP around the Zr(IV) atom (Table 2).
The total area (SVDP) of the surface of the VD polyhedron of zirconium atom [23.1(8) µ3 in the compounds studied is independent, within 2
s(SVDP) of the number of the fluorine atoms coordinated to the zirconium atom.The dimensionless second moment of inertia of the VD polyhedrons (G3), which is an integral parameter characterizing uniformity of the fluorine arrangement around the Zr atoms [8], is constant, within 2
s (G3), and equal to 0.082(1) independently of the C.N. and shape of the coordination polyhedron.The data [1] and the revealed lack of VVDP dependence on the C.N. and shape of the coordination polyhedron of Zr(IV) atom suggest that the Zr(IV) atoms surrounded by fluorine atoms in the crystal structures should be modeled not by the rigid spheres with fixed radii, but by the flexible spheres whose volume remains constant upon deformation under the action of the atoms of the first coordination sphere. In this connection, it is of interest to study the characteristics of the VD polyhedrons of the Zr(IV) atoms in the structure of the compounds containing coordination polyhedrons ZrOn.
Table 1. Main characteristics of the VD polyhedron of the Zr(1) atom in the Cs5Zr4F21 Ç3?2? structure [5]. The coordinates of the central atom Zr(1): x = 0.431, ? = 0.721, and z = 0.25, VVDP = 8.203 µ3 SVDP = 23.759 µ2 G3 = 0.0838256
Atom |
x |
y |
z |
ri(Zr-F), µ |
S„ % |
W i, % |
Q** |
Atom |
x |
y |
z |
ri(Zr-F), µ |
S„ % |
W i, % |
Q** |
F(l) |
0.401 |
0.825 |
0.052 |
2.019 |
15.00 |
15.22 |
5 |
F(4) |
0.556 |
0.781 |
0.078 |
2.150 |
12.76 |
12.45 |
5 |
F(l) |
0.401 |
0.825 |
0.448 |
2.019 |
15.00 |
15.22 |
5 |
F(4) |
0.556 |
0.781 |
0.422 |
2.150 |
12.76 |
12.45 |
5 |
F(3) |
0.289 |
0.660 |
0.250 |
2.043 |
13.27 |
14.12 |
4 |
*F(6) |
0.617 |
0.975 |
0.25 |
3.546 |
0.33 |
0.18 |
4 |
F(2) |
0.421 |
0.596 |
0.050 |
2.053 |
15.43 |
15.18 |
5 |
*F(7) |
0.668 |
0.691 |
0.25 |
3.713 |
0.03 |
0.01 |
4 |
F(2) |
0.421 |
0.596 |
0.450 |
2.053 |
15.43 |
15.18 |
5 |
Notes: * Labels the indirect neighbors of the central atom of the VD polyhedron.
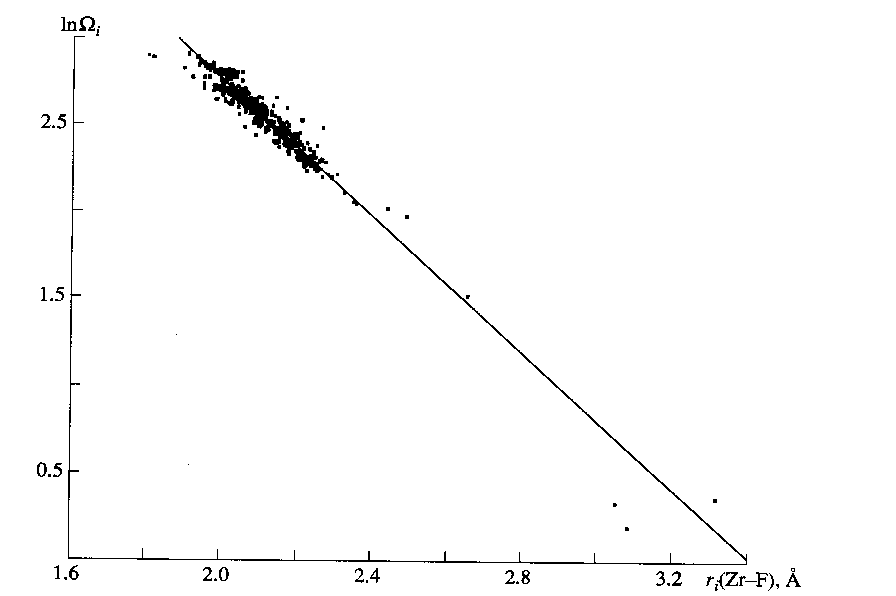
Fig. 2. Logarithm (lnW) of the solid angle corresponding to the face of the VD polyhedron of the Zr(IV) atom as a function of ri(Zr–F). The line corresponds to equation (1).
Table 2. Main characteristics of the VD polyhedrons of the Zr(IV) atoms surrounded by the fluorine atoms
C.N.Zr |
Number |
Characteristics of the Voronoi-Dirichlet polyhedrons |
||||
of compounds |
of types of atoms |
VVDP, µ3 |
Rsd, µ |
SVDP, µ2 |
G3 |
|
6 |
17 |
17 |
8.1(4) |
1.24(2) |
24.2(7) |
0.084(1) |
7 |
18 |
20 |
8.0(2) |
1.24(1) |
23.4(3) |
0.083(1) |
8 |
33 |
42 |
7.9(2) |
1.24(1) |
22.5(3) |
0.081(1) |
6,7 and 8 |
65 |
79 |
8.0(2) |
1.24(1) |
23.1(8) |
0.082(1) |
ACKNOWLEDGMENTS
This work was supported by the Russian Foundation for Basic Research, project no. 95-03-08583.
REFERENCES
1. Serezhkin, V.N„ Blatov, V.A., and Shevehenko, A.P., Koord. Khim., 1995, vol. 21, no. 3, p. 163.
2. Serezhkin, V.N., Shevehenko, A.P„ and Blatov, V.A., Koord. Khim., 1996, vol. 22, no. 1, p. 76.
3. Wells, A.F„ Structural Inorganic Chemistry, Oxford: Clarendon, 1984. Translated under the title Strukturnaya neorganicheskaya khimiya, Moscow: Mir, 1987, vol. 2, p. 164.
4. Blatov, V.A., Shevehenko, A.P., and Serezhkin, V.N., Zh. Strukt. Khim., 1993, vol. 34, no. 5, p. 183.
5. Tkachev, V.V, Davidovich, R.L., and Atovmyan, L.O., Koord. Khim., 1992, vol. 18, no. 1, p. 42.
6. Frank, F.C., Phase Transition in Metals and Alloys, New York: McGraw-Hill, 1967.
7. Blatov, V.A., Pol'kin, V.A., and Serezhkin, V.N., Kristallografiya, 1994, vol. 39, no. 3, p. 457.