Russian Journal of Coordination Chemistry, Vol. 22, No. 1, 1996, pp. 72-76. Translated from Koordinatsionnaya Khimiya, Vol. 22, No. 1, 1996, pp. 76-80. Original Russian Text Copyright � 1996 by Serezhkin, Blalov, Kuklina
Voronoi-Dirichlet Polyhedra of Uranium (ll-V) Atoms in Oxygen-Containing Compounds
V. N. Serezhkin, A. P. Shevehenko, and V. A. Blatov
Samara State University, Samara, Russia
Received May 11,1995
Abstract - Some features of the U(II-V) environment in oxygen-containing compounds are studied using the Voronoi-Dirichlet polyhedra (VDPs). The VDP volume of uranium atoms appears to be independent of the coordination number at a fixed oxidation state, and it regularly increases with a decrease in the oxidation state. The method of estimating the oxidation state of the uranium atoms in a crystal structure on the basis of geometric parameters of the VDPs is suggested. The advantages of applying the VDPs to determination of the coordination numbers of atoms in a crystal lattice are demonstrated by a number of examples.
The Voronoi-Dirichlet polyhedron (VDP) of atom A surrounded by atoms {Xi} is defined as a convex polyhedron whose surface is formed by the planes that pass through the midpoints of the A-Xi lines normal to them [1, 2]. In a crystal structure, for every crystallographic type of atoms, there is a VDP of a certain shape and size, which, in approximation [3,4], can be considered as a geometric image of the corresponding atom in the crystal field. The VDP and the coordination polyhedron of the same atom are dual (in particular, the number of the VDP faces is equal to the number of the vertices in coordination polyhedron and vice versa); therefore, the coordination number (CN) of the atom is equal to the number of faces of the corresponding VDP (CNVDP). For example, those U(VI) atoms that form in crystals coordination polyhedra of composition UOn (n = 6, 7, or 8), i.e., tetra-, penta-, or hexagonal bipyramids, respectively, correspond to the tetra-, penta-, or hexagonal-prism-shaped VDPs, respectively.
When analyzing the structures of the oxygen-containing U(VI) compounds [4], it was established that the volume of those VDPs that are dual to the coordination polyhedra of UOn is independent of the uranium coordination number and equal to 9.2(3) µ3 for 354 crystallographic types of the U(VI) atoms. In this connection, it is of interest to examine the effect of the uranium oxidation state on the geometric parameters of the corresponding VDPs, which was the prime objective of our work.
The computation of the VDPs of uranium atoms were performed with the DIRICHLET program, which is a part of the TOPOS structure-topological program package [5]. Initial information on the crystal structure of the U(II, III, IV, or V) compounds, which contain, according to the known structure description, UOn coordination polyhedra, was taken from the database for the structures of inorganic and coordination uranium compounds [6], which contains, at present, the data on 1028 compounds. Compared to the U(VI) complexes, the number of oxygen-containing U(II-V) compounds with the known structures is small; for this reason, we took into account all available crystal structural data (including earlier works, which used the photographic method of recording the diffraction pattern) with the divergence factor R < 0.15. At the same time, we did not consider the compounds with a fractional and/or ambiguous oxidation state of uranium atoms (as, e.g., in U3O8), as well as the compounds with statistical disordering of uranium and/or oxygen atoms. The data on 52 compounds, which contain 62 crystallographically independent types of U(II-V) atoms, fit the above requirements.
Table 1. Variation in the VDP volume (VVDP), VDP surface area (SVDP), radius of spherical domain (RSD) and volume of the coordination polyhedron (VCP) of uranium atoms with changing oxidation state (x ) and polyhedron composition
|
x (U) |
Polyhedron composition* |
Number of sorts of atoms U |
VVDP, µ3 |
SVDP, µ2 |
RSD, µ |
VCP, µ3 |
|
0 |
U12 |
3 |
20.8(1) |
40.5(4) |
1.705(3) |
73(5) |
|
U14 |
3 |
22.0(2) |
41.6(3) |
1.739(6) |
90(2) |
|
|
U15 |
1 |
23.3 |
42.8 |
1.77 |
100.7 |
|
|
II |
UO6 |
1 |
14.9 |
36.3 |
1.53 |
19.8 |
|
III |
UO9 |
1 |
12.6 |
30.0 |
1.44 |
32.0 |
|
IV |
UO6 |
7 |
12(1) |
31(2) |
1.41(5) |
24(9) |
|
UO8 |
15 |
11.1(4) |
28.2(6) |
1.38(2) |
23(1) |
|
|
UO9 |
3 |
11.1(3) |
27.9(6) |
1.38(1) |
27(1) |
|
|
UO10 |
10 |
11.2(6) |
28(1) |
1.39(3) |
31(2) |
|
|
UO11 |
1 |
11.6 |
28.4 |
1.40 |
36.5 |
|
|
UO12 |
11 |
11.1(5) |
27(2) |
1.38(2) |
41(7) |
|
|
V |
UO6 |
5 |
9.9(5) |
28(1) |
1.33(2) |
17(8) |
|
UO7 |
6 |
10.0(4) |
27.1(7) |
1.34(1) |
17.1(6) |
|
|
UO8 |
1 |
9.8 |
26.4 |
1.33 |
19.5 |
|
|
UO9 |
1 |
10.1 |
28.1 |
1.34 |
23.9 |
* In the presence of additional U-Xi contacts, the composition of the UOnXm polyhedra is written as UOn+m (if X = O) or UOn (if X
� O).
Table 2. VDPs of the uranium atoms in some compounds as calculated by the DIRICHLET program*
|
U2(CF3COO)8 Ç H2O Ç C18H36N2O6 [7] |
Ba0.98O3 [8] |
||||||
|
Central VDP atom U(l) VVDP = 11.37 µ3, |
Central VDP atom U(l) VVDP = 11.03 µ3 |
||||||
|
SVDP = 28.20 µ2 |
SVDP = 29.75 µ2 |
||||||
|
Atom X, |
r(U-Xi), µ |
Si, % |
W i, % |
Atom X, |
r(U-Xi), µ |
Si, % |
W i, % |
|
O(1) |
2.236 |
14.44 |
14.75 |
O(2) |
2.191 |
16.90 |
17.08 |
|
O(1) |
2.282 |
12.64 |
13.28 |
O(2) |
2.191 |
16.90 |
17.08 |
|
O(12) |
2.379 |
13.78 |
13.21 |
O(2) |
2.235 |
16.57 |
16.51 |
|
O(2) |
2.431 |
10.61 |
10.96 |
O(2) |
2.235 |
16.57 |
16.51 |
|
O(8) |
2.451 |
11.75 |
11.51 |
O(1) |
2.246 |
16.49 |
16.37 |
|
O(3) |
2.474 |
10.03 |
10.20 |
O1) |
2.246 |
16.49 |
16.37 |
|
O(4) |
2.501 |
10.56 |
10.38 |
Ba(1)** |
3.676 |
0.02 |
0.01 |
|
O(6) |
2.538 |
10.22 |
9.84 |
Ba(1)** |
3.676 |
0.02 |
0.01 |
|
O(7) |
2.718 |
5.94 |
5.86 |
Ba(1)** |
3.772 |
0.02 |
0.01 |
|
O(13)** |
3.527 |
0.02 |
0.01 |
Ba(1)** |
3.772 |
0.02 |
0.01 |
|
Ba(1)** |
3.919 |
*** |
*** |
||||
|
Ba(1)** |
3.919 |
*** |
*** |
||||
*The coordinates of the central atoms (U) of the VDPs and of the atoms that share faces with these VDPs (Xi), as well as some other characteristics, which are not used in this work, are omitted for simplicity. The area of the VDP face that is shared by a pair of the U and Xi atoms and the solid angle corresponding to this face (
W i) are expressed, for convenience, in percents of the total surface area of the uranium VDP (SVDP) and of the total solid angle of 4p sr, respectively. The notations of all atoms are as in [7,8].** The label that automatically denotes atom X, if the midpoint of the U-X; bond is not on the surface of the uranium VDP.
*** <0.01%.
Our results indicate that the number of faces in the VDP of uranium atoms in the sampled structures changes from 6 to 12, depending on the oxidation state (
x) of uranium atoms and on the composition and structure of a compound. This variation in the number of faces corresponds to the change in composition of the coordination polyhedron from UO6 to UO12 (Table 1). Note that, for 14 of 62 sorts of uranium atoms, the CNVDP exceeds the value reported in the original work (CNCLASS). A detailed analysis shows that the increase in CNVDP, compared to CNCLASS, is due to the presence of additional U-Xi contacts (from I to 6), which were not included in the traditional CN determination because these contacts were abnormally long from the classical point of view (>3 µ). In the structures under consideration, the role of the "additional neighbors" most often belongs to the nonmetal atoms (O or C), although, in two compounds (NaUO3 and Ba0.98UO3), the metal atoms (Na and Ba, respectively) were also found to be the additional neighbors.Analysis of the VDPs of the uranium atoms with CNVDP > CNCLASS indicates that the additional neighbors Xi correspond to the faces whose summarized area (Si) does not exceed 3% of the total surface of VDP (SVDP), and corresponding solid angles (
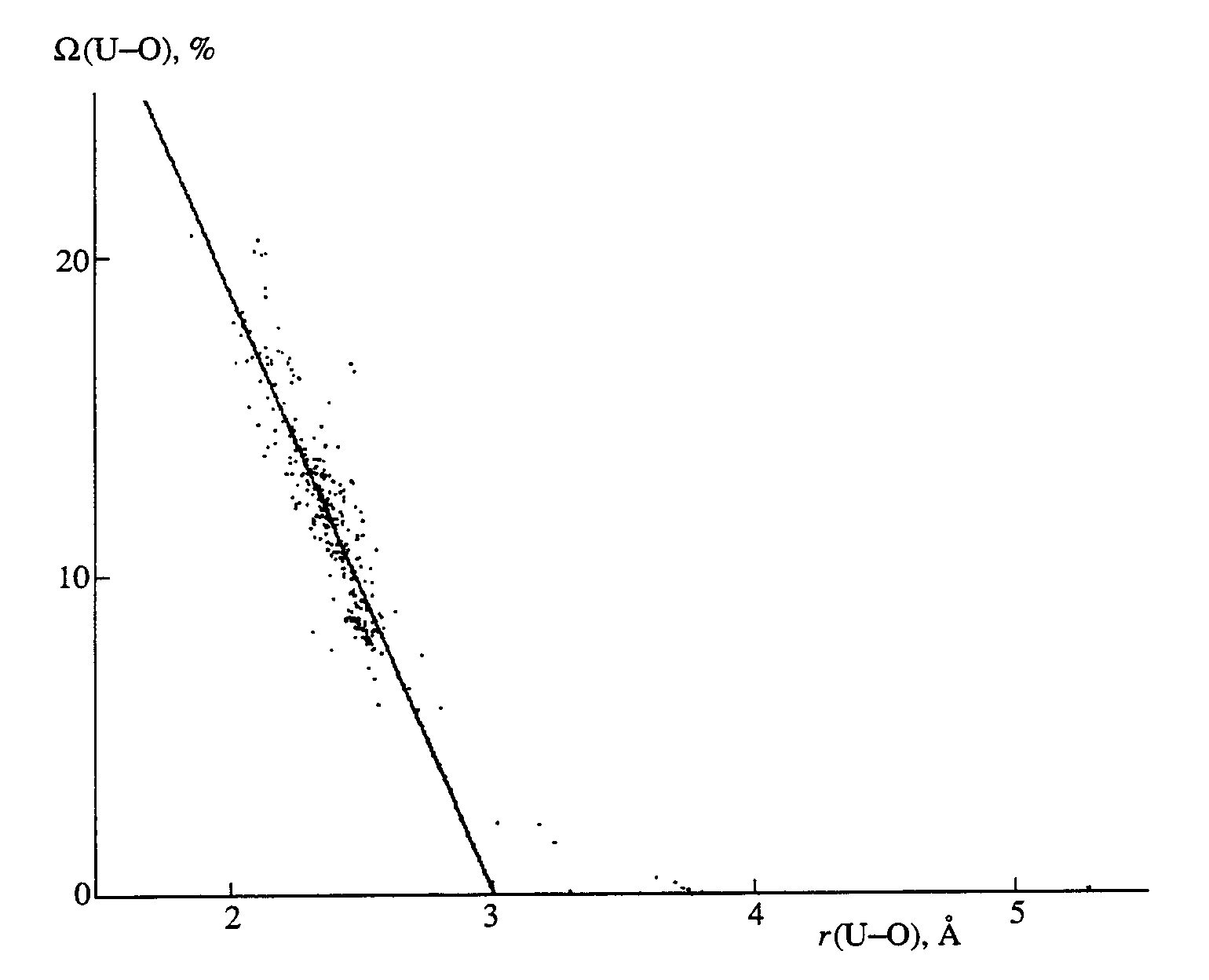
Wi) do not exceed 2.5% of the total solid angle 4p sr. As an example. Table 2 lists the parameters of the uranium VDPs in crystals U2(CF3COO)8 Ç H2O Ç C18H36N2O6 and Ba0.98UO3, whose structures were reported in [7, 8], respectively. Note that, for every face of the VDP of atom A, there is a solid angle Wi that is formed by the pyramid with atom A as an apex and face Si, as a base and is numerically equal to the area of the segment of a unit-radius sphere (centered at atom A) [3, 4]. In terms of our approach, we postulate that the bonding capacity of atom A is distributed over the A-Xi bonds with interatomic distances Ri, proportionally to the values of the solid angles Wi. If one assumes that Wi value for that face of the VDP of atom A that corresponds to the A-Xi contact is proportional to the fraction of the valence electrons of A that are involved in the formation of the A-Xi bond, then the additional interatomic U-Xi contacts for which Wi < 0.17 p sr and Si < 3% SVDP can be considered, according to [4], as nonbonded interactions.The distinction between the bonded and nonbonded U-Oi contacts is clearly demonstrated by the plots of solid angles
W i [or lnW i(U-O)] versus corresponding interatomic ri(U-O) distances. For the U(II-V) compounds, the relationship between W i and ri(U-O) (the figure) is described, according to linear regression analysis, by equationW
i (U-O) = 57(1) - 18.8(4)ri(U-O), (1)with the correlation coefficient p = -0.89 for 512 VDP faces with
W i > 1%. The remaining VDP faces with W i � l% were ignored when deriving equation (1).Dependence of the solid angles [W i(U-O), in percentages of the total solid angle 4p sr] on r(U-O) in 62 Voronoi-Dirichlet polyhedra of the U(II-V) atoms.
The lower limiting value for the considered
Wi quantities was taken to be equal to the triple maximum error of solid angle determination smax(W), which is caused by the errors of X-ray structural experiment. For the sampled compounds, smax(W) was ca. 0.3%. Note that, for the same 512 experimental points, the relationship between Wi and ri(U-O) can also be described, according to the least-squares method, by logarithmic dependence with r = -0.90:ln
Wi (U-O) = 6.65(9) - 1.77(4)ri(U-O). (2)In equations (1) and (2), the
W i(U-O) values are given in percentages of the total solid angle of the uranium atom, and ri(U-O) values are given in angstroms.Table 3. Characteristics of the VDPs of the uranium atoms in different oxidation states*
|
x (U) |
No. of the U atom sorts |
VVDP, µ3 |
SVDP, µ |
Rsd, µ |
VCP, µ3 |
|
0 |
7 |
22(1) |
41.3(9) |
1.729(25) |
84(11) |
|
II |
1 |
14.9 |
36.3 |
1.526 |
19.8 |
|
III |
1 |
12.6 |
30.0 |
1.442 |
32.0 |
|
IV |
47 |
11.2(6) |
28(2) |
1.388(26) |
30(9) |
|
V |
13 |
10.0(4) |
27.6(9) |
1.334(17) |
18(5) |
|
VI |
354 |
9.2(3) |
25.8(5) |
1.299(13) |
16(2) |
*For
x (U) = VI, the data are taken from [4].In our opinion, our data (Tables 1 and 3) allow one to conclude that, similarly to the U(VI) compounds, the VDP volume of the uranium atom with the fixed oxidation state (IV or V) and with the coordination polyhedron UOn (6
� n� 12) is independent, to within s, of its CN (i.e., of the n value). An increase in the uranium oxidation state (from two to six) is accompanied by a regular decrease in the volume of the corresponding VDP from 14.9 to 9.2 µ3. One can see from Table 3 that similar regularity is generally exhibited by the total VDP surface area. At the same time, contrary to VVDP, the volume of the corresponding coordination polyhedron considerably increases with an increase in the CN at a fixed oxidation state (Table 1), and its average value does not correlate with the uranium oxidation state (Table 3).When analyzing pair interatomic interactions, modern crystallochemistry, as a rule, deals with one-dimensional characteristics (atomic radii and interatomic distances); Table 3 presents the one-dimensional analog of the average VDP volume for each uranium valence state. As an analog, we used the radius of a spherical domain (RSD), which is numerically equal to the radius of the sphere whose volume is equal to the volume of the corresponding VDP. In other words, RSD characterizes the sphere into which an uranium VDP of arbitrary shape would transform if the oxygen atoms generated a spherically symmetrical mean crystal field. Interestingly, in three known modifications of metallic uranium, the average RSD value [1.73(3) µ, Table 3] coincides with the uranium Slater radius 1.75 µ to within
s [9]. A regular decrease in RSD with the increase in the oxidation state of uranium atoms (Table 3) may be considered, by analogy with the known fact that the effective ionic radius of atom A decreases with increasing x (A), as a result of the increase in the electron density transfer from the metal atom to the electron-accepting atoms of its first coordination sphere.An additional analysis showed that, for the U (IV-VI) atoms in the oxygen environment, the
x(U) values are linearly related to the RSD values,x
(U) = 29.17(7) - 17.88(5)RSD, (3)with the correlation coefficient -0.90.
It is of interest to consider the compounds with fractional oxidation states of uranium in terms of relationship (3). U3O8 is the classical example of such compounds. The question as to which of the two alternative formulas (specifically, U4+U26+O8 or U6+U25+O8) more adequately describes the valence state of the uranium atoms in the structure still remains to be solved [10]. By now, the structures of
a (at room temperature [II] and 500�C [12]) and b [13] modifications have been determined (structures 1, II, and III, respectively). All notations we used completely coincide with those used in [11-13], according to which structures I, II, and III contain two, two, and three crystallographically independent sorts of uranium atoms, respectively, with CN 6 or 7 and tetragonal- or pentagonal-bipyramidal coordination polyhedra, respectively. In this connection, note that the CNVDP coincides with CNCLASS for all uranium atoms, except for U(l) in structure II. The VDP of this atom shows the presence of the additional U(l)-O(3) contact (3.14 µ), which was disregarded in the classical description of II (Table 4); therefore, the CN of U(l) increases to 7 (or 6+1), and the coordination polyhedron of this atom should be considered as a pentagonal rather than tetragonal bipyramid.Note that, although the coordination polyhedra of two sorts of uranium atoms in structure I considerably differ from one another in the interatomic U-O distances for the equatorial plane of the UO7 pentagonal bipyramids (Table 4), the VDP volumes of these atoms are almost the same. It then follows from the preceding that the uranium atoms in structure I are in the same valence state. Contrary to I, the crystallographically inequivalent sorts of uranium atoms in structures II and III noticeably differ not only in the r(U-O) values, but also in the VVDP values (Table 4). Therefore, the uranium atoms in these structures can be considered to be in different valence states. Table 4 gives the values of
x (U) for the uranium atoms in structures I-III calculated by equation (3). Note that, although the value of VVDP of the uranium atoms in the three U3O8 structures changes over a rather wide range from 8.95 to 10.30 µ3, the mean value of VVDP of the uranium atom is virtually constant (9.65, 9.67, and 9.67 µ3 for I, II, and III, respectively).Table 4. Some characteristics of the uranium atoms in the structure of U3O8
|
Compound |
Atom |
N* |
R(V-O), µ, in the UOn polyhedron |
VVDP, µ3 |
RSD, µ |
x (U) |
|
I |
U(l) |
2 |
2.07, 2.07, 2.18, 2.18, 2.25, 2.25, 2.45 |
9.64 |
1.320 |
5.6 |
|
U(2) |
4 |
2.07, 2.07, 2.12, 2.16, 2.20, 2.21, 2.71 |
9.65 |
1.321 |
5.6 |
|
|
II |
U(l) |
2 |
2.07, 2.07, 2.12, 2.12, 2.21, 2.21, 3.14 |
9.93 |
1.333 |
5.3 |
|
U(2) |
4 |
2.07, 2.07, 2.15, 2.17, 2.18, 2.32, 2.49 |
9.54 |
1.316 |
5.6 |
|
|
III |
U(l) |
4 |
2.01, 2.08, 2.08, 2.28, 2.28, 2.40, 2.40 |
9.76 |
1.326 |
5.5 |
|
U(2) |
4 |
1.89, 1.89, 2.12, 2.30, 2.30, 2.37, 2.37 |
8.95 |
1.288 |
6.1 |
|
|
U(3) |
4 |
2.09, 2.09, 2.09, 2.09, 2.28, 2.28 |
10.30 |
1.350 |
5.0 |
* Multiplicity of the regular point system that is occupied by the uranium atoms.
In our opinion, this fact indicates that VVDP is an integral characteristic that is rather sensitive to a change in the valence state of uranium atoms, since in the compound with fixed chemical composition the increase in the oxidation state of some of the atoms (and, hence, the decrease in their VVDP or RSD) must be inevitably accompanied by an equivalent decrease in the oxidation state (i.e., an increase in VVDP or RSD) of the other part of the atoms. Therefore, it is not surprising that, although the calculated
x (U) values in structures I-III change from 5.0 to 6.1 (Table 4), their summarized values per formula unit U3O8 are almost the same (16.8, 16.5, and 16.6, respectively). In our opinion, this is in satisfactory agreement with the theoretical value 16. In I-III, s (RSD) � s (R(U-O)) � 0.02 µ, and the error in determining x (U) by equation (3) is about 0.5. This fact and the data in Table 4 allow one to suggest that, in the structures of the U3O8 modifications, 5 � x (U)� 6; for I and II, the formula should be written as U35.33+O8, and for III, U6+U25+O8. Note that this inference is consistent with the results of quantum chemical calculations of the electron-density disproportionation in uranium oxygen-containing compounds (including U3O8), which were recently obtained in terms of the Xa -SW method [14].Therefore, our results indicate that geometric parameters of the VDPs can be successfully used for determining the coordination numbers of the metal atoms in the inorganic and coordination compounds. It is significant that, contrary to the conventional method of determining the coordination numbers, the suggested approach does not require consideration of the data on crystallochemical atomic radii because, in this approach, the chemical A-X bond is thought to exists if the VDPs of atoms A and X share a face. Moreover, the correlation found between the solid angles of the VDP faces corresponding to the pair interatomic U-O interactions and the appropriate interatomic distances in the UOn polyhedra allows one to unambiguously and simultaneously determine not only strong (bonded) but also weak (nonbonded or additional) interatomic interactions, which have been the object of current crystallochemical investigations [15-17]. The invariance of the volume of the Voronoi-Dirichlet polyhedra that are dual to the classical UOn coordination polyhedra with various uranium coordination numbers, which was found by the example of oxygen-containing compounds, with regard to [18] allows us to assume that, in addition to the commonly accepted classical model of a crystal as a packing of hard spheres, one should take into account the alternative geometric model of the crystal structure as a packing of soft (or deformed) spheres. In this connection, it is of some urgency to carry out a systematic analysis of the properties of the VDPs of the atoms in the structures of various classes of inorganic and coordination compounds that differ in the nature of both complex-forming atoms A and coordinated donor atoms X.
ACKNOWLEDGMENTS
This work was supported by the Russian Foundation for Fundamental Research, project no. 95-03-08583.
REFERENCES