The two simplest with R = H and CH3 are glycine and alanine.
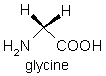
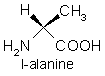
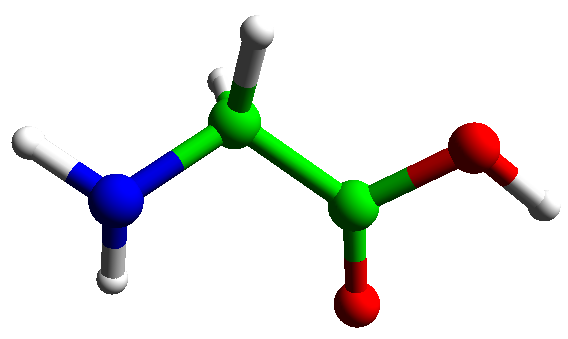
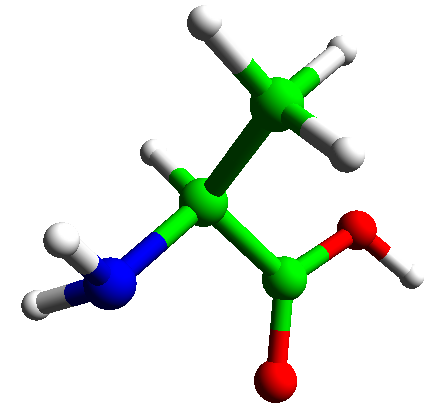
Two amino acids may be joined together by loss of water to form a dipeptide. The bond formed in this way is called a peptide bond.


Proteins contain many amino acid joined in this way. A chain joined by peptide links defines the primary structure of a protein.
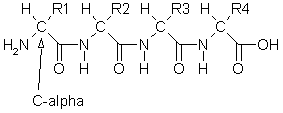
Along the chain the R groups attached to each alpha carbon (Ca) are all that changes from the amino (-NH2) end to the carboxylic (-COOH) end. The secondary and tertiary structure of proteins depend upon the way these chains coil up and fold. There are two examples of proteins in the moilin library, crambin and dsv flavodoxin.
Crambin is a relatively small seed storage protein from a plant called abyssinian cabbage it has 46 residues (parent amino acids). To make it easy to see these large molecules C-alpha only pictures can be used.
This is the crambin C-alpha

On the left you can see a helical section of what is called alpha-helix.
This is perhaps easier to see rotated 90 degrees.

All the non-H atoms of crambin give a complex picture however the yellow S-S links are visible.

dsv flavodoxin is an electron transfer protein and it has 147 residues. Its electro active part is flavin mono nucleotide or FMN. Its C-alpha+FMN
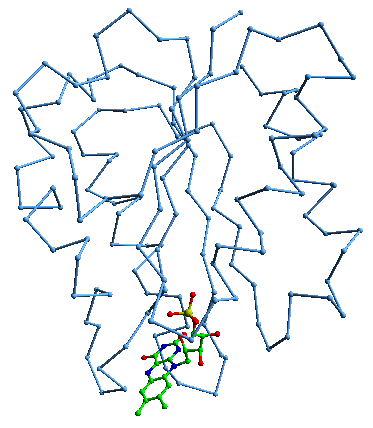
is a lot easier to understand than the full picture.

You can just make out the FMN on the bottom left.
Back